Key Considerations for Contract and Component Manufacturers while entering Europe
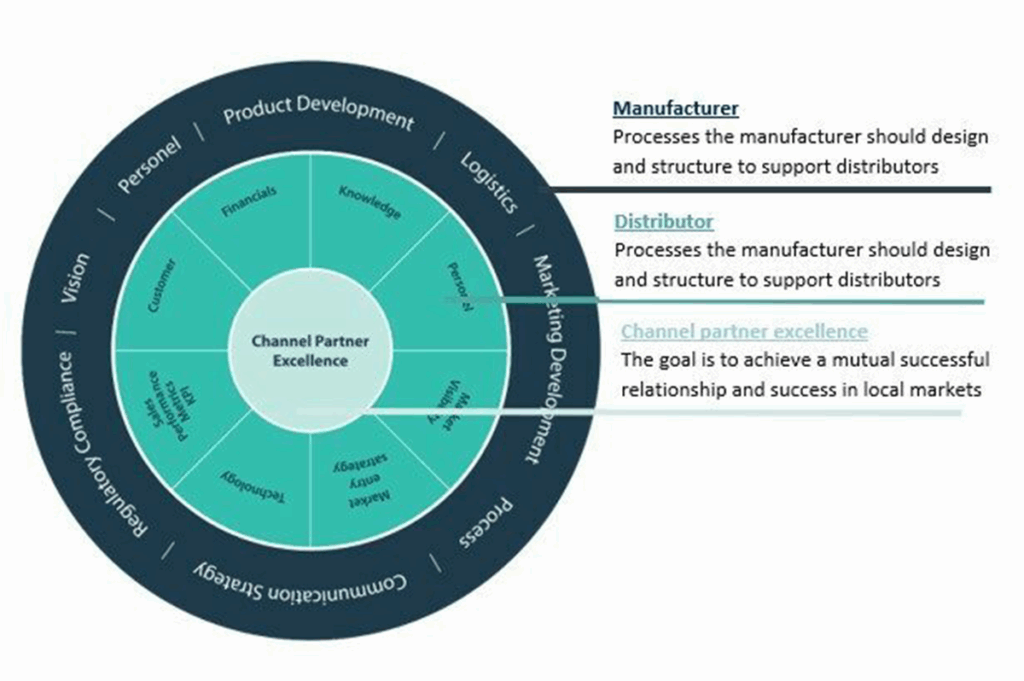
Embarking on the journey to enter the European OEM market as a contract or component manufacturer in the medical industry? Careful planning and strategic considerations are essential for navigating the diverse cultural and commercial landscape in Europe. Here’s what to watch out for when stepping into the realm of European medical manufacturing: Key considerations: Regulatory Compliance: CE Marking within the MDR/IVDR regulations are for finished goods and does often not apply for CMO’s. However European OEMs do require preferably the ISO 13485 certificate at a minimum and the requirement to perform audits themselves in the manufacturing plant. GMP Compliance: Good Manufacturing Practice (GMP) compliance is essential for ensuring the quality and safety of medical products. Adhering to GMP standards is a prerequisite for market entry in Europe. Market Research: Understand Local Demands: The European market is diverse, with varying manufacturing needs across countries. Conduct thorough market research to understand the specific demands and preferences in your target regions. Understand your competitors capabilities, their lead times and how they are serving the OEM market, in which market segments. Localization of products: Consider adapting your products to meet local requirements and languages. Localization enhances acceptance and relevance in different European markets. There are other metrics and there may be other requirements. Flexibility in production to serve the EU market is key. Distribution & Logistics: Make sure your logistical team is aware of the shipping processes to be prepared for flawless shipping. For larger components and parts its good to sign a contract with a European carrier. The shipping lead times should be considered in the delivery process as lead times remain an important supplier criteria. Consider warehousing options strategically to optimize supply chain efficiency. Building a trusted brand: As a new supplier in the European market you would need to build trust. Because you exist 10-20-30 years in your own market doesnt make you a trusted partner in Europe. Creating trust in the market means presenting yourself as a CMO with European service, support. It means building relationships and raising brandawareness consistently for example by exhibiting or visiting tradeshows regularly. Having a European office, with European sales support in the time-zone, in various languages, having documentation translated, the website translated, having a European phone-line and staff that understands the market can make the difference. Collaborate with local expert: Engage with local consultants, regulatory experts, and industry associations. Their insights and guidance can prove invaluable in navigating the complexities of the European market. Understand Tariffs & taxes: Familiarize yourself with import/export tariffs and taxes applicable to your products. A sound financial strategy is essential for sustainable operations in the European market. Entering the European market as a contract or component manufacturer in the medical OEM industry demands a strategic and informed approach. Tops events taking place in 2024 in Europe: MedtecLIVE Location: Stuttgart, Germany Date: 18-20 June 2024 Focus: MedtecLIVE is a premier event that brings together the entire medical technology supply chain. From components and materials to manufacturing processes, it provides a comprehensive overview of the industry landscape. COMPAMED Location: Düsseldorf, Germany Date: 11-14 November, 2024 Focus: As a part of the MEDICA trade fair, COMPAMED focuses specifically on high-tech solutions for medical technology. It is a hub for suppliers of components, manufacturing, and contract services. Medtech Ireland Location: Galway, Ireland Date: 25-26 September, 2024 Focus: Medtech is a premier tradeshow in Ireland dedicated to medical components. It provides a comprehensive overview of the latest technologies, manufacturing processes, and components driving advancements in the medical sector. The event attracts a diverse audience, including manufacturers, suppliers, engineers, researchers, and healthcare professionals To see the full list of all European healthcare tradeshows check out our blog here. GrowthMedics specializes in providing market development services for CMO’s looking to enhance their sales in European medical markets, from catheters, plastics, micro-molding, coating to instruments, implants, wearables and others. Reach out to us to learn about our capabilities and how we can elevate your growth in Europe! & check our industry expertise in the contract and component manufacturing industry by clicking here. Let’s meet at MD&M 2024 to to discuss the potential of your product & services in the European Market. Click here to book a meeting with us.